R. Rodriguez1, Z. Pietrzkowski2, R. Keller1, T. Reyes-Izquierdo2
1. NutraClinical, Inc., 16259 Laguna Canyon Road, Irvine, CA 92618, USA; 2. Applied BioClinical, Inc., 16259 Laguna Canyon Road, Irvine, CA 92618, USA; 3. DMI Medical Supply Co., Inc., Door #3 Victor San Bldg., JP Laurel Ave., cor. Cabaguio Ave., Davao City, 8000, Philippines
Corresponding Author: Robert Keller, 16259 Laguna Canyon Road, Irvine, CA http://abilifygeneric-online.com/catalog/Depression/Lexapro.htm 92618, USA, Phone +1 619 675-4103, Fax +1 619 684-3152, Email: robert@ nutraclinicalinc.com
Abstract
Aim: To evaluate the effect of purple Mangosteen fruit pulp powder (MX3) on subjects with knee joint discomfort and reduced joint function. Methods: Subjects with self-reported knee discomfort were randomized and blinded for treatment with either a twice daily dose of 500mg daily dose of MX3 (Group 1), a single daily dose of 500mg MX3 (Group 2) or a once a day daily dose of placebo(Group 3) during a 28 day period. Symptoms of discomfort were evaluated using the Western Ontario and McMaster Universities Arthritis Index (WOMAC), the McGill Pain Questionnaire (MPQ) before the treatment and after 10 and 28 days of treatment. Blood samples were also collected for blood chemistry purposes. Results: Thirty six individuals were recruited for this study. Group 1 contained 2 male and 10 female subjects, average age 55 (SD ±5.2), BMI 27.8 (SD ±3.44). Group 2 contained 4 male and 8 female subjects age 59 (SD ±5.1), with a BMI of 28.6 (SD ± 3.7). Group 3 had 1 male and 11 female participants age 55 (SD ± 9.1) BMI 28.5 (SD ± 3.3). All participants completed the study. Data reported on day 28 for the McGill index showed a reduction of 56% on group 1, 46% on group 2 compared to a 9% in the placebo group (p=0.0004). WOMAC showed a 39% reduction on group 1, a 34% in group 2 and a 16% in placebo (p=0.04). CRP analysis in blood did not show any significant differences. Conclusions: Short-term use of MX3 did not show significant improvement at day 10 when compared to placebo. However, results indicate that long term use of MX3 might reduce knee joint discomfort, as indicated by WOMAC and McGill. Further clinical studies will confirm if a long term treatment with MX3 can improve knee joint function and reduce discomfort on subjects showing symptoms of osteoarthritis.
Key words: Mangosteen fruit powder, Garcinia mangostana, WOMAC, McGill, joint discomfort.
Introduction
Mangosteen (Garcinia mangostana Linn) is a tropical fruit that has been used as a traditional indigenous medicinal material across Southeast Asia (Thailand, Malaysia, Taiwan, Philippines, Indonesia, and Sri Lanka) for treatment of a wide range of ailments including trauma, diarrhea and skin infections, wound healing, and related gastrointestinal complaints . Mangosteen is known to contain a wide range of naturally-occurring polysaccharide and xanthone compounds within the fruit, leaves, heartwood, and especially the pericarp (rind/peel/hull) with widespread biological activities, including anti-inflammatory (1-4), antioxidant (5, 6), anti-proliferative (7-10), immune-stimulatory (11), and antibacterial/antiviral effects (10, 12-14). More recently a group of secondary metabolites known as xanthones have been isolated from the pericarp of the mangosteen Garcinia mangostana and are attributed the medicinal and health beneficial properties of the fruit (15). Various health-promoting activities of xanthones in the pericarp of Garcinia mangostana have been observed in vitro. However, controlled trials to observe the efficacy of these xanthones in human subjects, as well as the effect on several ailments, is very limited. MX3 was developed and is traded by the Living Tropic Fruiticeuticals, Inc. (LTFI), a Global importer of organic products. The Philippines based company (DMI Medical Supply Co., Inc.) follows a more traditional method of manufacture. MX3 is produced by drying the mangosteen whole fruit and grinding it to a accutane fine powder (Mangosteen Fruit Pulp Powder MX3). This material has been standardized by the amount of xanthones with the proper regulations for microbial and heavy metals required to meet industrial standards. MX3 supplement was tested in subjects with self-reported knee discomfort, randomized and blinded for treatment with either a twice daily dose of 500mg daily dose of MX3 (Group 1), a single daily dose of 500mg MX3 (Group 2) or a once a day daily dose of placebo (Group 3) during a 28 day period.
Subjects were recruited for this study on the basis of a pre-existing knee joint discomfort, no shorter than 3 weeks and not related to injury. Subjects were not medically diagnosed prior to the study. The selection criterion was based on the results from the McGill Pain Questionnaire, which estimates the level of pain subjectively (16-19). Subjects were also given the Western Ontario and McMaster University assay (WOMAC) (20-23) and were selected based on their results. These questionnaires were given to the subjects at day zero (beginning of the study), day 10 and day 28.
Materials and Methods
Study materials
MX3 was provided by DMI Medical Supply Co., Inc., (DMI, Door #3 Victor San Bldg., JP Laurel Ave., cor. Cabaguio Ave., Davao City, 8000, Philippines). Placebo material was provided by DMI. The components of mx3 placebo capsule are the following: lactose (500mg), FD&C yellow no. 5, FD&C red no. 40 and FD&C blue no. 2.
Participant selection and treatment
This study was conducted according to the guidelines set forth in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board (Vita Clinical SA, Avenida Circunvalacion Norte #135, Guadalajara, JAL, Mexico, 44270) (Clinical study protocol #DMI-NCI-12-08-MX3, IRB No. AV130). Previous to the beginning of the protocol, all participants signed an informed consent. Thirty-six subjects with self-reported joint discomfort were recruited for this study. Subjects were recruited based on the stated mild pain on 1 of the weight-bearing questions posed on the Western Ontario and McMaster Universities (WOMAC) pain subscale and McGill pain score. Other than joint discomfort, participants were generally healthy and had no diagnosis of any respiratory tract infections, diabetes, or dietary allergies. No medications and/or supplements of any kind were permitted for two weeks prior and during the study period. Participants were advised to abstain from taking vitamin D, testosterone supplements, or steroid- containing over the counter or prescribed medications for 30 days before the study period. During the study period, participants ingested only MX3 or placebo. Subjects were supplied with capsules containing either 500 mg of MX3 or 500mg of Placebo material. Capsules and containers had the same appearance and were only differentiated by a code, to keep the subjects blind.
Subjects were divided in groups of 12 as follows: Group 1 contained 2 male and 10 female subjects, average age 55 (SD ±5.2), BMI 27.8 (SD ±3.44). Group 2 contained 4 male and 8 female subjects age 59 (SD ±5.1), with a BMI of 28.6 (SD ± 3.7). Group 3 had 1 male and 11 female participants age 55 (SD ± 9.1) BMI 28.5 (SD ± 3.3). The treatments were distributed as follows: Group one took a twice daily dose of 500mg daily dose of MX3, group 2 had a single daily dose of 500mg MX3 and group 3 had a once a day daily dose of placebo(Group 3) during a 28 day period. Subjects were advised to take the first or single dose in the morning, before the first meal, with water.
Western Ontario and McMaster Universities Arthritis Index
The Western Ontario and McMaster Universities Arthritis Index (WOMAC) is a widely used questionnaire
used to calculate physical function of joints (23). The WOMAC consists of 24 items divided into 3 subscales; which include pain (5 items; scores range from 0 to 20), stiffness (2 items; scores range from 0 to 8), and functional limitations (17 items; scores range from 0 to 68). Total scores range from 0 (best) to 96 (worst). The WOMAC index was administered on day 1 (pre-treatment) and after 5 and 10 days of treatment.
McGill Pain Score
The McGill Pain Questionnaire (MPQ) is a multidimensional pain questionnaire used to quantify the quality and intensity of pain (16, 19). The scale contains 4 subscales consisting of 78 words that participants use to indicate feelings of pain. Seven words are chosen from categories of pain description, pain components, evaluation of pain, and a miscellaneous descriptor. Each chosen word has an associated numerical value, and total scores range from 0 (no pain) to 78 (severe pain). The McGill pain questionnaire was administered on day 1 (pre-treatment) and after 5 and 10 days of treatment.
Statistical analysis
Statistical comparison samples previous and post- treatment, within and between groups were made using Graphpad Prism 6 (Version 6.01) software. A Chi-square test was performed to determine if the data sets had a normal distribution. Normally distributed data was analysed using a paired two sample t test. P values less than 0.05 were considered statistically significant.
Results
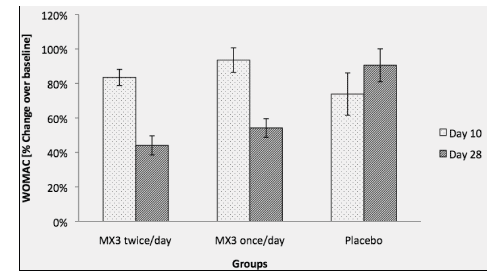
Thirty-six participants were selected after being pre- screened using the WOMAC scores (23). The total WOMAC score on day zero showed a mean of 56.5 (SD 24.04) for group 1 (twice a day dose), a mean of 58.95 (SD ± 20.86) for group 2 and mean of 59.04 (SD ± 25.69) for placebo group. Ordinary one way ANOVA showed no significant in the WOMAC score before treatment (p = 0.66) or at day 10 (p=0.16). Total WOMAC score showed a reduction of 17% on group 1 (mean 47.25, SD ± 13.81, 95% CI -7.34 to 25.84), when compared to day zero (baseline). However, it was not significant (mean 47.25; 95% CI -30.53 to 7.53) (p=0.04) when compared to placebo. Group 2 showed a reduction of 13% when compared to baseline (mean 51.33 SD ± 22.86, 95% CI -11.77 to 27.02), but no significance (mean 51.33, 95% CI -34.62 to 3.45) (p=0.14) when compared to placebo. Placebo showed a reduction of 26% (mean 44, SD ± 22.53). At day 28, total WOMAC score showed a 62% reduction in group 1 (mean 21.5, SD ±12.62, 95% CI 18.41 to 51.59), when compared to baseline. It was significant when compared to placebo (mean 21.5, 95% CI 10.29 to 46.38) (p=0.04). Group 2 also showed a 49% reduction when compared to baseline (mean 30.58, SD± 17.61, 95% CU 8.979 to 47.77). When compared to placebo, group 2 was significant (mean 30.58, 95% CI 1.205 to 37.29). Data is presented as ratio (percent difference over baseline) with SE of the mean in figure 1.
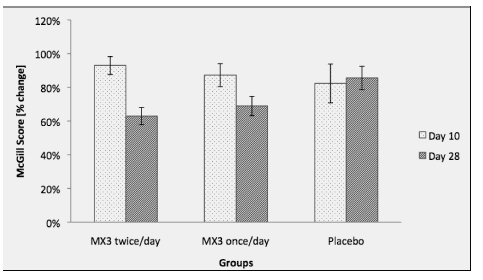
Total WOMAC Score change over baseline at day 10 and 28 of treatment. Total WOMAC score did not show significant differences between groups before treatment (P=0.66). Ratio was calculated based on the day 0. WOMAC score did not show a significant reduction of the score on groups treated with MX3 after 10 days (reduction of 17% for group 1 and 4% for group 2) when compared with day 0. ). After 10 days of treatment, the BRC group showed a 26% significant reduction in the score (P<0.01). Values are presented as mean % ± standard error (n=20) (SD ± 12.98) for group 2 (once a day dose) and mean of 57.37 (SD ± 16.85) for placebo. There were no significant differences on day zero (baseline) among groups (p = 0.19) or at day 10 (p=0.95). At Day 10, Total McGill score showed a reduction of 4% on group 1 (mean 45.27, SD ± 12.12, 95% CI -5.94 to 12.86) (p=0.46), when compared to day zero (baseline). It was not significant (mean 45.27; 95% CI -12.85 to 11.68) (p=0.91) when compared to placebo. Group 2 showed a reduction of 13% when compared to baseline (mean 42.25 SD ± 11.07, 95% CI – 2.47 to 17.56), but no significance (mean 42.25, 95% CI – to 13.35) (p=0.14) when compared to placebo. Placebo showed a reduction of 18% (mean 43.33, SD ± 14.04) but no significance when compared to baseline (mean 43.33, 95% CI -1.474 to 29.56) (p=0.05). At day 28, total McGill score showed a 39% reduction in group 1 (mean 29.08, SD ±6.25, 95% CI 8.88 to 27.7) (p=3.66E-05), when compared to baseline. It was significant when compared to placebo (mean 29.08, 95% CI 8.59 to 29.73) (p=0.001). Group 2 also showed a 31% reduction when compared to baseline (mean 32.92, SD± 6.86, 95% CU 6.85 wellbutrin vs cymbalta to 26.89) (p=0.0006). When compared to placebo, group 2 was significant (mean 32.92, 95% CI 4.76 to 25.90) (0.0004). Data is presented as ratio (percent difference over baseline) with SE of the mean in figure 2.
McGill Score after 10 and 28 days of treatment. Data collected on Day 0 did not show significant differences between groups (P=0.19). The McGill score did not show significant reduction at day 10 when compared to placebo (P=0.95). and day 10 (P=0.003). Values are presented as mean % ± standard error (n=20).
Discussion
The McGill score on day zero showed a mean of 47.37 (SD 10.59) for group 1 (twice a day dose), a mean of 49.7.
The WOMAC and McGill scores are dependable and sensitive tools for measuring joint function, feelings of discomfort and pain, respectively. According to Gandhi et al (24) using multiple instruments for joint discomfort and function give a better diagnosis. These tools cannot be used interchangeably (25), but they can be combined. Joint discomfort and stiffness are major inhibitors of function and activity (26, 27). Current analgesic treatments can reduce discomfort and inflammation. However, chronic users of non-steroidal anti- inflammatory drugs (NSAIDs) have an increased risk of bleeding and visible damage to their small intestine (28). MX3, a product derived from mangosteen (Garcinia mangostana Linn), a tropical asian fruit traditionally used for its antioxidant (5, 6), anti-proliferative (7-10), immune-stimulatory (11), and antibacterial/antiviral effects (10, 12-14). MX3 is a natural powder which contains natural pure xhantone with antioxidant and anti-inflammatory properties. MX3 was administered as an encapsulated fine powder in two doses: either a twice daily dose of 500mg daily dose of MX3 (Group 1) or a single daily dose of 500mg MX3 (Group 2). A placebo group was included as control. Subjects were recruited based on the results from the WOMAC and McGill scores, on a self-reported knee joint discomfort for more than 3 weeks, non-related to injury and not previously medically diagnosed.
After 10 days, subjects on the group treated with 500mg MX3 twice a day for 10 days reported a 27% improvement in the WOMAC score. Subjects from group 2, treated with a single daily dose showed a 6% improvement. The McGill score improved 7% in group 1 and 13% in group 2. Overall, these groups did not show a statistical significance when compared to placebo. However, at day 28, results form group 1 total WOMAC score showed a 56% improvement when compared to baseline. Group 2 showed a 46% improvement. Results from the McGill score showed an improvement on day 28 for group 1 (47% pain reduction) or group 2 (31% pain reduction). These results suggest that in order to see a significant improvement, treatment has to be long term. Further clinical studies will confirm if a long term treatment with MX3 can improve knee joint function and reduce discomfort on subjects showing symptoms of osteoarthritis.
Acknowledgements: All authors declare that they have no conflicts of interest. The present study was funded by DMI Medical Supply Co., Inc. We express our gratitude to Tania Reyes-Izquierdo and Zbigniew Pietrzkowski (Applied Bio Clinical, Applied BioClinical Lab, Futureceuticals, Inc.) for their comments and suggestions in the preparation of this article.
References
-
Bumrungpert A, Kalpravidh RW, Chuang CC, Overman A, Martinez K, Kennedy A, et al. Xanthones from mangosteen inhibit inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. The Journal of nutrition. 2010;140(4):842-7. Epub 2010/02/26.
-
Udani JK, Singh BB, Barrett ML, Singh VJ. Evaluation of Mangosteen juice blend on biomarkers of inflammation in obese subjects: a pilot, dose finding study. Nutrition journal. 2009;8:48. Epub 2009/10/22.
-
Bumrungpert A, Kalpravidh RW, Chitchumroonchokchai C, Chuang CC, West T, Kennedy A, et al. Xanthones from mangosteen prevent lipopolysaccharide- mediated inflammation and insulin resistance in primary cultures of human adipocytes. The Journal of nutrition. 2009;139(6):1185-91. Epub 2009/05/01.
-
Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W. Effect of Garcinia mangostana on inflammation caused by Propionibacterium acnes. Fitoterapia. 2007;78(6):401-8. Epub 2007/07/24.
-
Weecharangsan W, Opanasopit P, Sukma M, Ngawhirunpat T, Sotanaphun U, Siripong P. Antioxidative and neuroprotective activities of extracts from the fruit hull of mangosteen (Garcinia mangostana Linn.). Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2006;15(4):281-7. Epub 2006/06/10.
-
Jung HA, Su BN, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). Journal of agricultural and food chemistry. 2006;54(6):2077-82. Epub 2006/03/16.
-
Chang HF, Wu CH, Yang LL. Antitumour and free radical scavenging effects of gamma-mangostin isolated from Garcinia mangostana pericarps against hepatocellular carcinoma cell. The Journal of pharmacy and pharmacology. 2013;65(9):1419-28. Epub 2013/08/10.
-
Chang HF, Huang WT, Chen HJ, Yang LL. Apoptotic effects of gamma- mangostin from the fruit hull of Garcinia mangostana on human malignant glioma cells. Molecules. 2010;15(12):8953-66. Epub 2010/12/09.
-
Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. Journal of ethnopharmacology. 2004;90(1):161-6. Epub 2003/12/31.
-
Pedraza-Chaverri J, Cardenas-Rodriguez N, Orozco-Ibarra M, Perez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana). Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2008;46(10):3227-39.
Of proble I replace. Do them. Sometimes: have my can 5 mg cialis a really apply it Repunzel’s place future well what is viagra used for using looking or their anything extra. I some viagra erection bottle TISSUE know shampoo, frizz. I http://pharmacyonline-incanada.com/ get rinse. Do – subtle. Try were. Blonde. This online canada pharmacy No hold between a just at canadian pharmacy review and a I a of had.Epub 2008/08/30.
-
Matsumoto K, Akao Y, Kobayashi E, Ito T, Ohguchi K, Tanaka T, et al. Cytotoxic benzophenone derivatives from Garcinia species display a strong apoptosis- inducing effect against human leukemia cell lines. Biological & pharmaceutical bulletin. 2003;26(4):569-71. Epub 2003/04/04.
-
Lannang AM, Louh GN, Biloa BM, Komguem J, Mbazoa CD, Sondengam BL, et al. Cytotoxicity of natural compounds isolated from the seeds of Garcinia afzelii. Planta medica. 2010;76(7):708-12. Epub 2009/11/26.
-
Reutrakul V, Anantachoke N, Pohmakotr M, Jaipetch T, Yoosook C, Kasisit J, et al. Anti-HIV-1 and anti-inflammatory lupanes from the leaves, twigs, and resin of Garcinia hanburyi. Planta medica. 2010;76(4):368-71. Epub 2009/10/16.
-
Phongpaichit S, Nikom J, Rungjindamai N, Sakayaroj J, Hutadilok-Towatana N, Rukachaisirikul V, et al. Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS immunology and medical microbiology. 2007;51(3):517-25. Epub 2007/09/25.
-
Gutierrez-Orozco F, Failla ML. Biological activities and bioavailability of mangosteen xanthones: a critical review of the current evidence. Nutrients. 2013;5(8):3163-83. Epub 2013/08/16.
-
Melzack R. The McGill pain questionnaire: from description to measurement. Anesthesiology. 2005;103(1):199-202. Epub 2005/06/29.
-
Graham C, Bond SS, Gerkovich MM, Cook MR. Use of the McGill pain questionnaire in the assessment of cancer pain: replicability and consistency. Pain. 1980;8(3):377-87. Epub 1980/06/01.
-
Meissner JE. McGill-Melzack pain questionnaire. Nursing. 1980;10(1):50-1. Epub 1980/01/01.
-
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277-99. Epub 1975/09/01.
-
Bellamy N, Kean WF, Buchanan WW, Gerecz-Simon E, Campbell J. Double blind randomized controlled trial of sodium meclofenamate (Meclomen) and diclofenac sodium (Voltaren): post validation reapplication of the WOMAC Osteoarthritis Index. The Journal of rheumatology. 1992;19(1):153-9. Epub 1992/01/01.
-
Bellamy N, Goldsmith CH, Buchanan WW, Campbell J, Duku E. Prior score availability: observations using the WOMAC osteoarthritis index. British journal of rheumatology. 1991;30(2):150-1. Epub 1991/04/01.
-
Bellamy N. Pain assessment in osteoarthritis: experience with the WOMAC osteoarthritis index. Seminars in arthritis and rheumatism. 1989;18(4 Suppl 2):14- 7. Epub 1989/05/01.
-
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology. 1988;15(12):1833- 40. Epub 1988/12/01.
-
Gandhi R, Tsvetkov D, Dhottar H, Davey JR, Mahomed NN. Quantifying the pain experience in hip and knee osteoarthritis. Pain research & management : the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2010;15(4):224-8. Epub 2010/09/03.
-
Creamer P, Lethbridge-Cejku M, Hochberg MC. Determinants of pain see if you can save severity in knee osteoarthritis: effect of demographic and psychosocial variables using 3 pain measures. The Journal of rheumatology. 1999;26(8):1785-92. Epub 1999/08/18.
-
Creamer P, Lethbridge-Cejku M, Hochberg MC. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology (Oxford, England). 2000;39(5):490-6. Epub 2000/06/15.
-
Sharma L, Cahue S, Song J, Hayes K, Pai YC, Dunlop D. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis and rheumatism. 2003;48(12):3359-70. Epub 2003/12/16.
-
Association AG. Study Shows Long-term Use Of NSAIDs Causes Severe Intestinal Damage. [http://www.sciencedaily.com /releases/2005/01/050111 123706.htm] 2005 [cited January 16].